Pompe disease is a genetic disease caused by GAA gene mutation. The activity of acid glucosidase in the patient's body is reduced or almost inactivated, resulting in glycogen degradation disorder, which is stored in the muscle liver and causes a series of severe symptoms until it threatens life. According to the age of onset, disease progression and disease severity, it can be clinically divided into infantile Pompe disease (IOPD) and late-onset Pompe disease (LOPD). IOPD is a dangerous disease that seriously endangers the life of children. The current clinically available therapeutic drugs cannot meet the long-term medical needs of Pompe disease patients, especially cannot improve the clinical dilemma of IOPD.
GC301 adeno-associated virus injection is a gene therapy drug. The active ingredient is a recombinant adeno-associated virus vector carrying the GAA gene expression sequence. Through a single intravenous infusion, it reaches all organs of the body at one time, achieving the purpose of long-term improvement of GAA enzyme activity level, continuous removal of accumulated glycogen and restoration of muscle strength. International similar products are all designed and developed for LOPD.
Not only Pompe disease, nowadays, as the genetic causes of more rare diseases are clarified, patients with rare diseases have the opportunity to obtain accurate diagnosis results, and patients with rare diseases have become a group widely recognized, concerned and understood by the society. Not giving up on every patient with a rare disease is the determination of the country and society. Promoting China's rare disease gene drugs from basic to clinical and market is the long-term persistence of the Jinlan team for many years. We believe that with the joint efforts of all sectors of society, the problem of rare disease treatment will be solved faster. To date, GeneCradle has promoted three candidate drugs for gene therapy of rare diseases into the clinical trial review stage. Their clinical indications are spinal muscular atrophy, genetic defective hypertriglyceridemia, and Pompe disease, which has been accepted this time. We also look forward to their real entry into the clinic in the near future and bringing real benefits and improvements in life to patients.
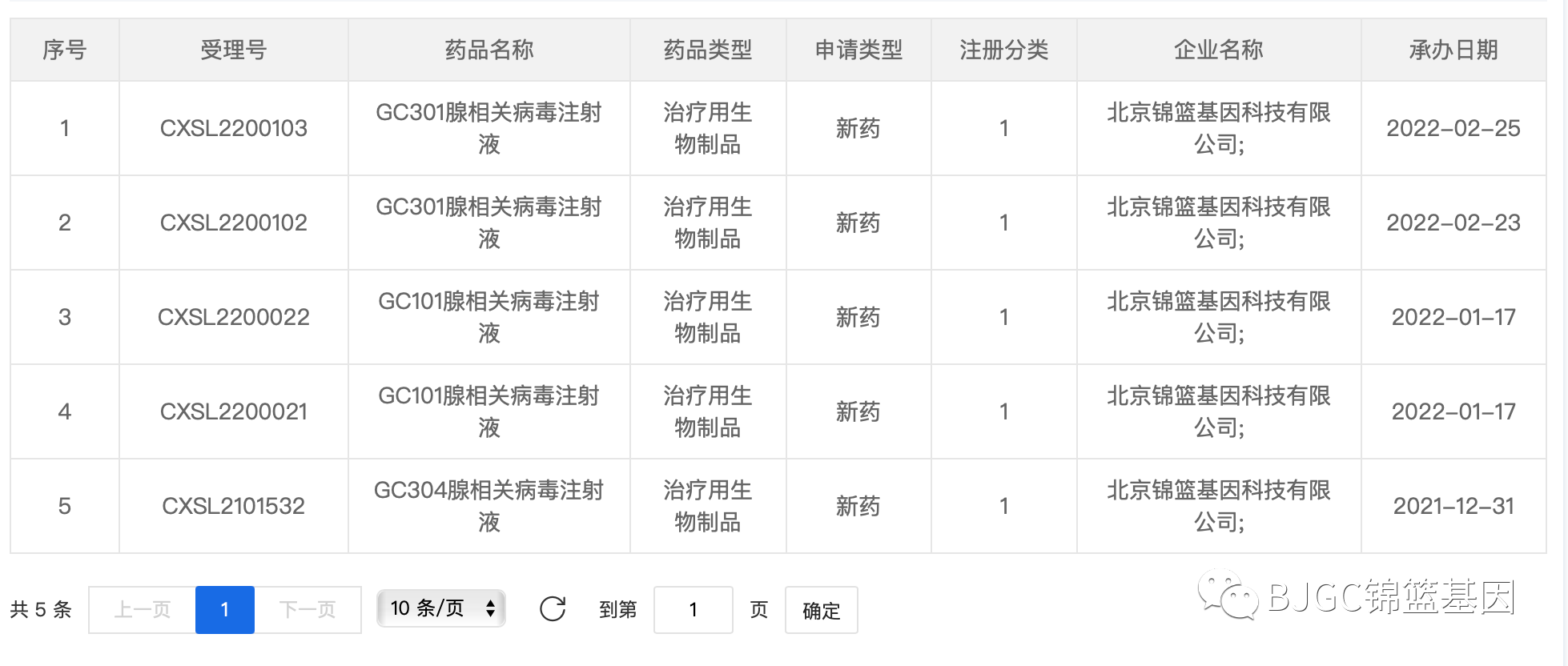
▲ Image source: Screenshot of CDE official website



