On December 11, 2024, following the conclusion of the public notice period by the Center for Drug Evaluation (CDE) of the National Medical Products Administration of China, Genecradle Therapeutics' independently developed GC101 injection was officially included in the list of breakthrough therapy varieties. The indication is for Type 2 spinal muscular atrophy (5q SMA), marking a milestone breakthrough in the genetic treatment of SMA in China.
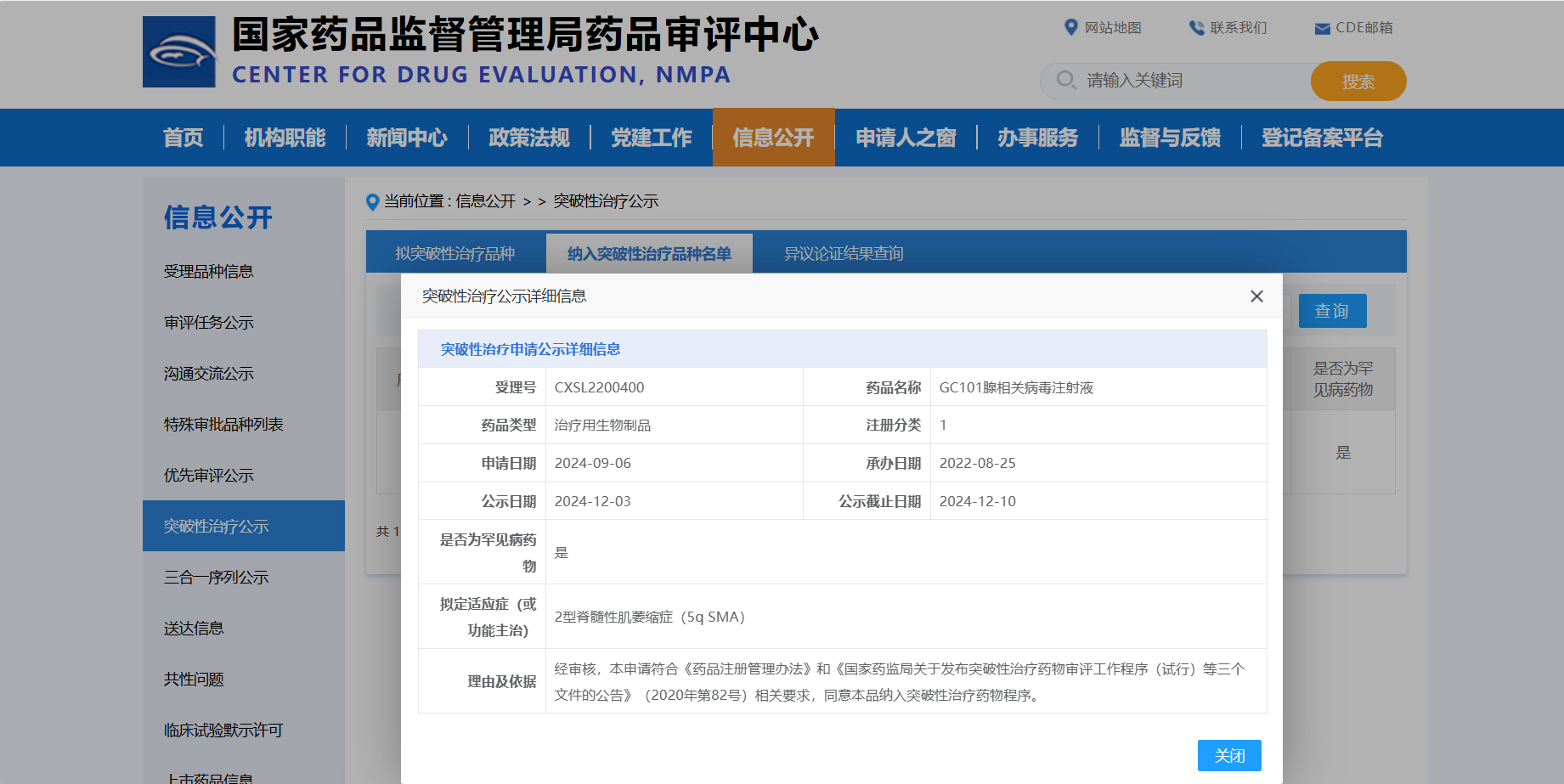
Image Source: Screenshot from the official CDE website
Breakthrough therapy designation, as an important mechanism within the national drug regulatory system, specifically refers to innovative drugs that target diseases that seriously threaten life or severely affect patients' quality of life, and for which there are currently no effective treatment methods or that show significant clinical advantages over existing therapies. This designation aims to accelerate the research and marketing process of such drugs through a fast-track approval channel, to meet the urgent clinical needs of patients as soon as possible.
GC101 injection is the first and only domestically developed AAV gene therapy product for intrathecal administration to treat SMA, with indications including Type 1 SMA, Type 2 SMA, and Type 3 SMA. Clinical trial data have shown significant and breakthrough therapeutic effects in Type 2 SMA subjects treated with GC101 injection, achieving multiple new motor milestones. Compared to existing treatment methods, GC101 injection has demonstrated more significant therapeutic effects, realizing the breakthrough of "one-time treatment, long-term efficacy."
The inclusion of GC101 injection in the list of breakthrough therapy varieties is an important milestone in the drug development journey of Genecradle Therapeutics, which will accelerate the drug's marketing process, allowing more SMA patients to benefit from this advanced therapy sooner. This recognition not only brings welfare to SMA patients but also affirms the innovative R&D strength of Genecradle Therapeutics. In the future, Genecradle Therapeutics will continue to adhere to its original intention and policy of "originating from needs, benefiting patients," commit to exploration and innovation in the field of gene therapy, continuously promote the progress of medical science and technology, and provide more efficient and safe treatment plans for patients with genetic diseases, creating a healthy future together.
About GC101 Adeno-Associated Virus Injection
GC101 adeno-associated virus injection is an AAV gene therapy that delivers a recombinant serotype 9 adeno-associated virus vector (rAAV9) carrying a normal SMN1 gene expression cassette through a one-time intrathecal injection, enabling the expression of the SMN1 gene in motor neuron cells, thereby improving the function of affected cells such as motor neurons. With the assistance of respiratory and motor rehabilitation training, it can improve and enhance the respiratory capacity and motor ability of SMA patients. The indication covers Type 1, Type 2, and Type 3 spinal muscular atrophy. Clinical trials have shown significant safety and efficacy, achieving the breakthrough of "one-time treatment, long-term efficacy."
About Spinal Muscular Atrophy
Spinal muscular atrophy is the leading genetic cause of infant mortality, a hereditary neuromuscular disease caused by mutations in the survival motor neuron gene 1 (SMN1), leading to functional deficiencies in SMN protein. The carrier frequency of the pathogenic gene in the Chinese population is about 1 in 42, with an incidence rate of about 1 in 10,000 in newborns. Type 1 SMA presents significant muscle weakness at birth or within the first few days of life. By six months of age, the symptoms are quite pronounced. Infants with reduced muscle tone and reflexes have difficulty sucking, swallowing, and breathing. Type 2 SMA patients mostly lose the ability to sit independently and become paralyzed during adolescence, often dying from complications such as respiratory failure; Type 3 SMA patients are affected by long-term medication use and gradual loss of motor ability, impacting their normal life.
About Genecradle Therapeutics
Beijing Genecradle Therapeutics Technology Co., Ltd. is a national high-tech enterprise with core business in the development of gene therapy drugs mediated by AAV vector delivery technology. The company is committed to promoting Chinese rare disease gene drugs from the foundation to clinical and market applications, benefiting patients and families. The company focuses on the development of gene therapy drugs for genetic neuromuscular diseases, inherited metabolic diseases, lysosomal diseases, and ophthalmic diseases, and through advancing the research and clinical application of rare disease gene drugs, it deepens the understanding of life and health, transitioning gene therapy technology and products from rare diseases to the treatment and rehabilitation of chronic and other major diseases.



