
The "Yinian Conference and Project Application Guide Release Conference" was successfully held in Beijing on March 18, 2023. The conference was hosted by Beijing Yicheng Cooperation and Development Foundation, and invited many well-known experts in the field of rare disease treatment in China to share the cutting-edge results of clinical research on rare diseases in China. GeneCradle's two gene therapy drugs GC101 injection and GC301 injection were deeply shared by many experts at the conference as new scientific and technological research and development achievements in the field of rare diseases.
Professor Qiu Zhengqing from the Department of Pediatrics, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences was invited to share with the whole society the current status of gene therapy for Pompe disease at home and abroad and the progress of scientific and technological achievements. Professor Qiu mainly introduced Pompe disease and the current treatment status, and focused on the development of gene therapy for Pompe disease and the gene therapy product from GeneCradle - GC301 injection. Professor Qiu introduced in detail the advantages of GC301 injection in clinical application from the perspective of a clinical expert. Professor Qiu said that GC301 injection is the fastest-progressing gene drug at present, and it is also the first internationally cutting-edge gene drug for infantile Pompe disease to obtain clinical trial approval. It has completed the administration of 3 patients in the researcher-initiated clinical study (IIT) conducted at the Seventh Medical Center of the General Hospital of the People's Liberation Army. The longest follow-up time has reached 187 days, showing good safety and tolerability, and the patients' heart and motor function have benefited significantly. GC301 injection will carry out IND clinical trials in the Department of Pediatrics, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences.



Professor Qiu Zhengqing's online live broadcast
Professor Xiong Hui from the Department of Pediatrics of Peking University First Hospital and Professor Feng Zhichun from the Department of Pediatrics of the Seventh Medical Center of the General Hospital of the Chinese People's Liberation Army were also invited to share their scientific and technological achievements in the new progress of spinal muscular atrophy research and early diagnosis and treatment of spinal muscular atrophy. According to the two experts, two drugs for spinal muscular atrophy have been launched in China. With the advent of the era of gene therapy, patients are eager to have an affordable and long-acting gene therapy drug suitable for Chinese patients on the market. As a representative gene therapy drug for spinal muscular atrophy in China, GeneCradleGC101 injection has shown its leading and superior performance in terms of technological innovation and clinical effects. Professor Feng Zhichun from the Department of Pediatrics of the Seventh Medical Center of the General Hospital of the Chinese People's Liberation Army said that since the first patient was enrolled and given the drug, GC101 injection has shown good results in terms of safety and effectiveness. At present, the IND project of GC101 injection has been fully launched, and patients will be able to use affordable gene therapy drugs developed by Chinese people earlier in the future.


Professor Xiong Hui's online live broadcast


Professor Feng Zhichun's online live broadcast
Tao Mingjun, director of Qingdao Brain Disease Rehabilitation Hospital, which focuses on the rehabilitation treatment of rare diseases, also shared his experience. Director Tao pointed out that in order to better promote the recovery of motor function in SMA patients, patients need to receive scientific muscle training and other rehabilitation treatments after treatment. At the same time, Director Tao said that since GeneCradle's gene drugs are administered once and are effective for a long time, they reduce the pain of multiple drug injections for patients and reduce the concerns of patients' parents about the increase in oral drug use as they age. This has a good role in promoting the rehabilitation of patients with rare diseases and enhancing the confidence of patients' families.

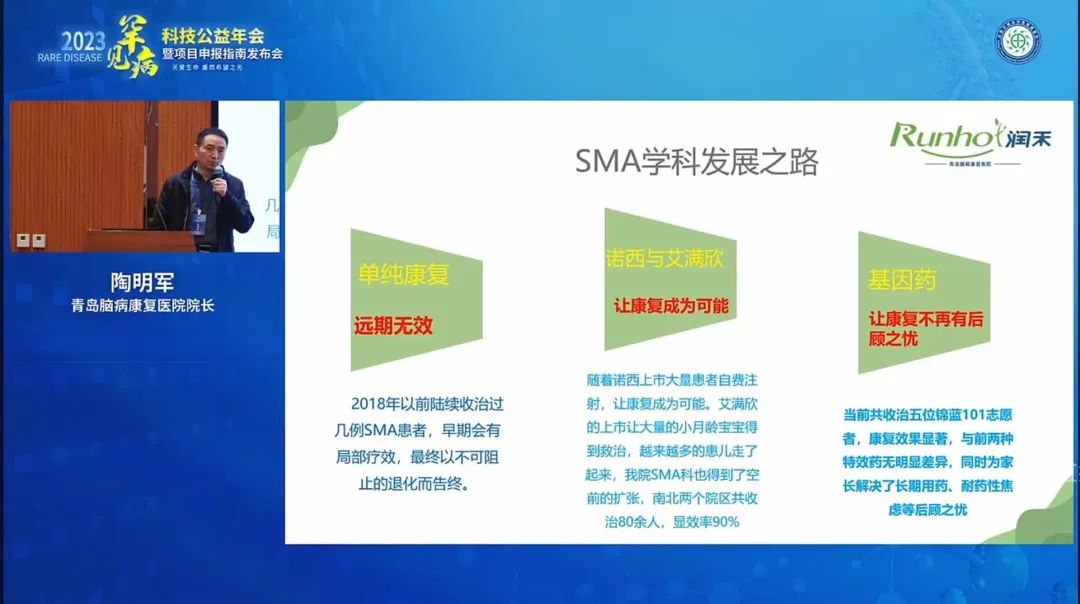
Dean Tao Mingjun's online live broadcast
GeneCradle has been continuously contributing to the public welfare of rare disease science and technology. As Professor Zou Liping, director of the Academic Committee of the Department of Pediatrics of the PLA General Hospital, said: "It is the love and responsibility behind science and technology that change the fate of patients with rare diseases." In the future, GeneCradle will actively promote the scientific and technological achievements of rare diseases from research and development to the market, promote the accessibility of gene drugs in China, and provide better treatment options for more patients with rare diseases.
About GeneCradle
Beijing GeneCradle Technology Co., Ltd. is a national high-tech enterprise with AAV vector delivery technology-mediated gene therapy drug development as its core business. Its mission is to promote China's rare disease gene drugs from basic to clinical and market, benefiting patients and families. The company focuses on the development of gene therapy drugs in the fields of hereditary neuromuscular diseases, genetic metabolic diseases, lysosomal diseases and ophthalmic diseases. By promoting the development and clinical application of rare disease gene drugs, it has a deeper understanding of life and health, and has transitioned gene therapy technology and products from rare diseases to the treatment and rehabilitation of chronic diseases and other major diseases.



