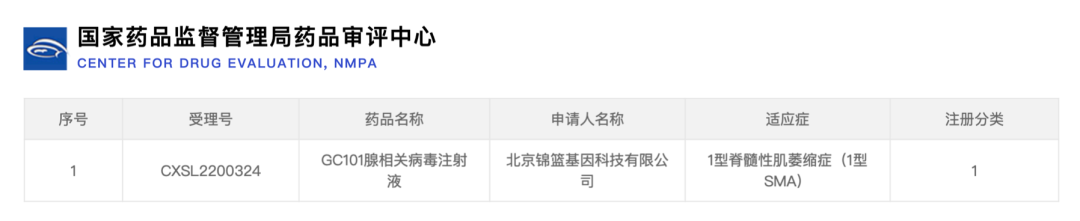
The Center for Drug Evaluation of the National Food and Drug Administration accepted the clinical trial registration applications for GC101 injection for type 1 SMA and type 2 SMA on July 19, 2022 and August 25, 2022, respectively. The clinical program that first completed the review and obtained implicit permission to conduct clinical trials is the first AAV gene therapy program in China and abroad to use intrathecal administration to intervene in the treatment of type 1 SMA.

About Spinal Muscular Atrophy
Spinal muscular atrophy (SMA) is a hereditary neuromuscular disease caused by a mutation in the survival motor neuron 1 (SMN1) gene, which results in a defect in the function of the SMN protein. It is one of the autosomal recessive genetic diseases that causes infant mortality and has been included in the "First List of Rare Diseases" jointly issued by the National Health Commission and five other departments. The incidence of the disease is between 1/8000 and 1/11000. Gene mutations cause the SMN protein level in SMA patients to decrease to varying degrees, affecting neuronal function and causing neurogenic atrophy of the patient's muscles. There is still a large unmet clinical need for this disease.
About GC101 Injection
GC101 Injection is designed to treat 5q spinal muscular atrophy. It delivers and supplements SMN protein directly to the central nervous system through intrathecal administration to improve the respiratory function and motor ability of SMA patients. GC101 Injection was studied in the early stage by Beijing Ruixi Rare Disease Gene Therapy Technology Research Institute. Beijing Wujiahe Gene Technology Co., Ltd. and its subsidiary Beijing Jingpudi Technology Co., Ltd. completed product production and pharmaceutical research. Beijing GeneCradle Technology Co., Ltd. completed pharmacological research, clinical program formulation and drug registration application, and will carry out subsequent clinical research and clinical trials.
Beijing GeneCradle Technology Co., Ltd. is a biotechnology platform company focusing on the innovative research and development of gene therapy drugs. It focuses on the research and development of gene therapy products in the fields of hereditary neuromuscular diseases, genetic metabolic diseases, lysosomal diseases and ophthalmic diseases mediated by AAV vector delivery technology. Its mission is to promote China's rare disease gene drugs from basic to clinical and market, and is committed to turning "incurable" into "once and for all" cures, benefiting patients and their families. Through the research and development and clinical application of rare disease gene drugs, we have a deeper understanding of life and health, and transition to the treatment and rehabilitation of chronic diseases and other major diseases.



